
Our Patients
1 in 10 individuals is affected by autoimmune disease
The increasing prevalence of autoimmune diseases has become one of the leading concerns in public health. Autoimmune diseases occur when the immune system mistakenly attacks healthy cells and tissues in the body, leading to chronic inflammation, tissue damage, and organ dysfunction. As more people are diagnosed with autoimmune disease, the burden on healthcare systems, families, and communities grows.

Resetting the body’s response for individuals with autoimmune disorders
At Artax Biopharma, we are working on a novel approach to address autoimmune diseases by modulating immune responses, not inhibiting them. Autoimmune diseases arise when the body loses its ability to distinguish between pathogenic antigens and self, thereby attacking the body’s cells as if they were pathogens. With our first-in-class drug candidates, we aim to modulate, or reset, the body’s immune response so that recognition of self is more normal again, while maintaining the ability to fight true pathogens.
We aim for a universal oral therapy with broad application in autoimmune diseases
We are searching for a universal oral therapy to treat autoimmune disease: a pill to address a wide range of autoimmune diseases, without causing the immune suppression that drives so many unwanted side effects and opportunistic infections. We observed strong preclinical evidence of activity in the Th2, Th17, Th1/Th0 pathways, suggesting potential use across the autoimmune space, which was confirmed in a Phase 2a clinical trial in patients with psoriasis.
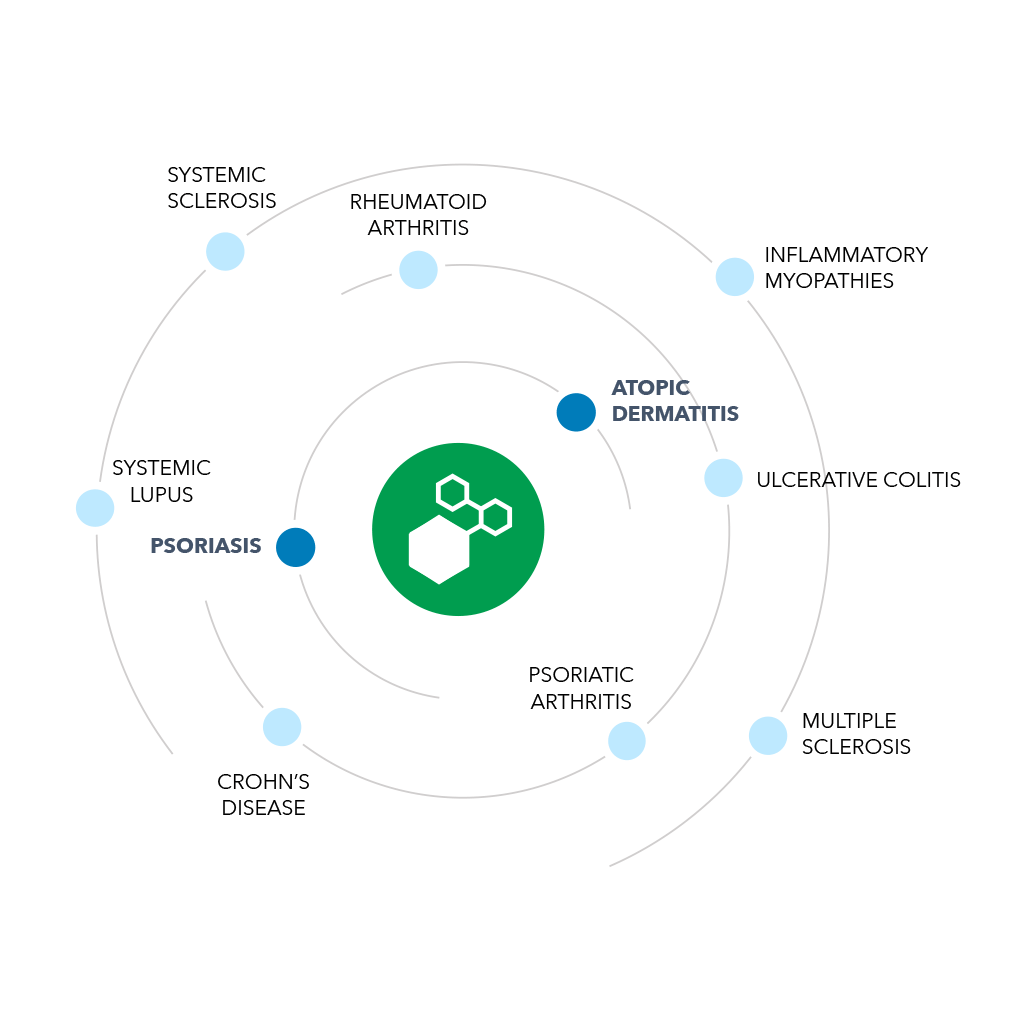
About our clinical trials
- Phase 2a clinical trial of AX-158 in psoriasis patients
Phase 2a clinical trial of AX-158 in psoriasis patients
AX-158 was evaluated in a Phase 2a trial in people with mild to moderate plaque psoriasis in multiple centers in the UK. Participants were randomized 2:1 to receive a single daily dose of 10mg AX-158 or placebo. A total of 30 participants were treated for 28 days and followed for an additional 30 days for safety. The main objectives of the study were safety and proof of mechanism. Learn more about the trial design here.
The Phase 2a study confirmed the well-tolerated safety profile seen in the Phase 1 studies, with no infections reported and no severe adverse events. The few adverse events reported were mild or moderate in nature and resolved without intervention, and there were no trial discontinuations.
An extensive psoriasis biomarker analysis was designed, conducted, and analyzed by Dr. James Krueger, Head of Laboratory for Investigative Dermatology at the Rockefeller University and member of the Scientific Advisory Board at Artax Biopharma. His analysis demonstrates statistically significant and consistent effects on both disease and pathway-related genes in participants treated with AX-158.
The company reported the results at the Society for Investigative Dermatology 2025 Annual Meeting – see links to our press release and poster.